top of page
LAWS
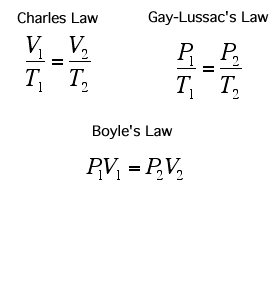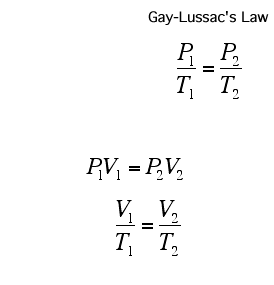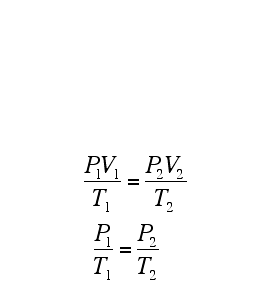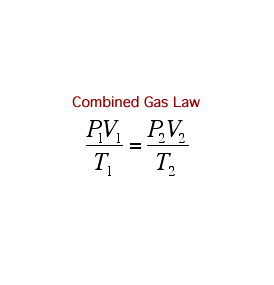
Dalton's law of multiple proportions says that these chemicals will present themselves in proportions that are small whole numbers (i.e. 1:2 O:H in water); although in many systems (notably biomacromolecules and minerals) the ratios tend to require large numbers, and are frequently represented as a fraction. Such compounds are known as non-stoichiometric compounds.
Chemical laws are those laws of nature relevant to chemistry. The most fundamental concept in chemistry is the law of conservation of mass, which states that there is no detectable change in the quantity of matter during an ordinary chemical reaction.
What It Means

bottom of page